Phosphorous & Orthophosphate
Similar to nitrogen, phosphorous serves as a key component of living systems, with multiple chemical forms and sources, the most common found in nature being phosphates, PO43-. Phosphates occur in three forms: orthophosphate, metaphosphate, and organically bound phosphate. While there are specific differences between each form, the primary difference between phosphate and orthophosphate is that orthophosphate is composed of only one phosphate unit, whereas phosphate can be composed of any number of phosphate units.
Phosphorous Inputs:
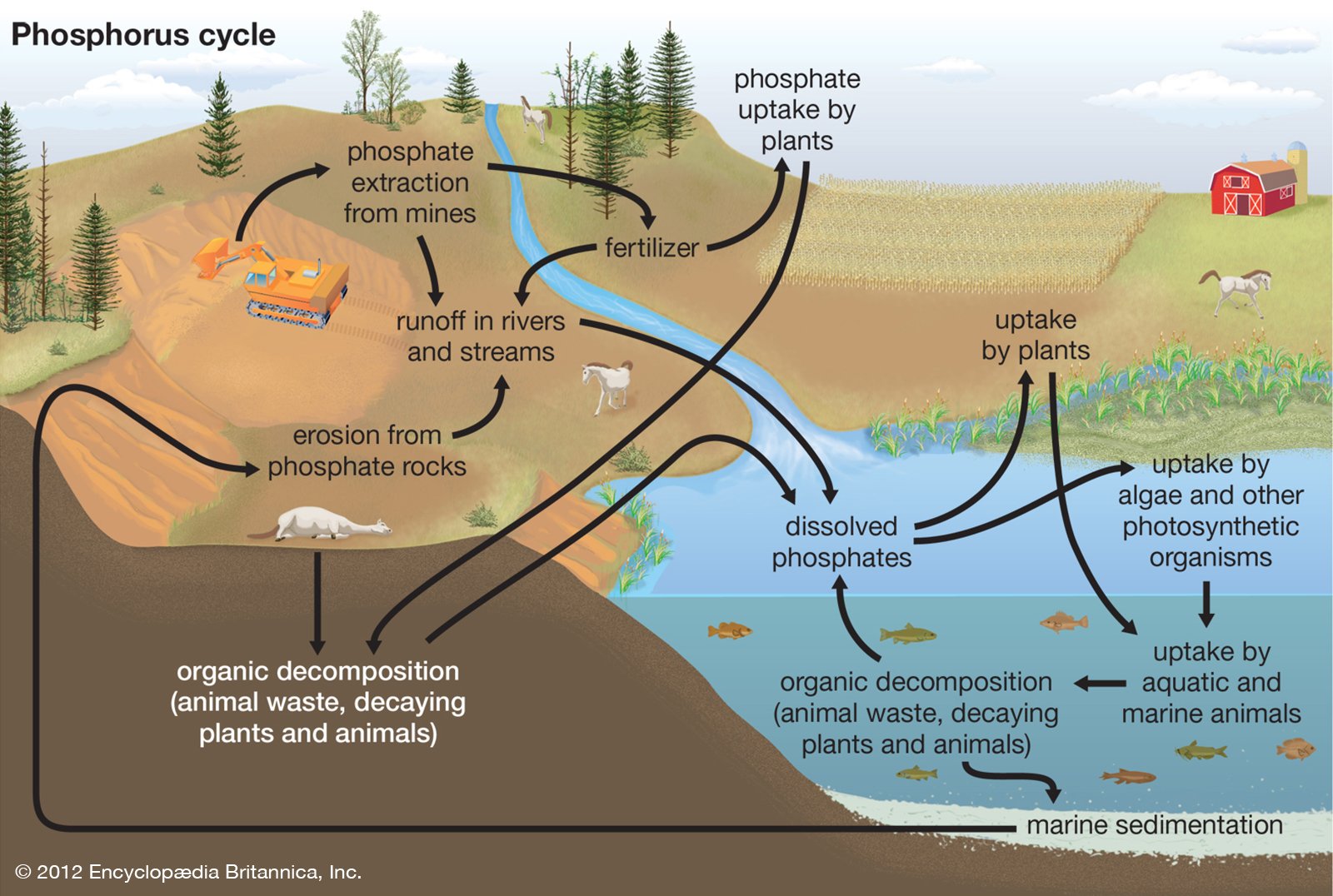
Understanding phosphorous in general, the natural cycle itself is slow compared to other cycles (think: water, carbon, nitrogen), occurring moreso within geologic timescales, as most natural phosphorous input comes from the weathering of sedimentary rocks. This, coupled with the crucial biologic role phosphorous plays (in cellular respiration, photosynthesis, and metabolism), often makes it the limiting nutrient, or nutrient that is least available in aquatic ecosystems. However, more and more excess phosphorous has been observed in aquatic ecosystems, leading to serious water quality consequences. Here, we will explore the natural and anthropogenic (human-originated) phosphorous inputs to a water system.
The phosphorous cycle with natural and anthropogenic inputs. Image source: The Encyclopedia Britannica.
Natural Phosphorous:
The natural phosphorous cycle is relatively slow, determined by both biologic (biotic consumption and excretion) and geologic (the speed at which rocks weather, and sediments are deposited) factors. Biotically, phosphate compounds found in the soil are taken up by plants, which are consumed by animals. When plants or animals die or produce waste, the phosphorous is returned to the soil or bacteria within, and some of this is washed to rivers, lakes, and oceans, where it becomes available to aquatic organisms. When these marine organisms produce waste or die, the resulting compounds sink to the floor of the ocean, where they settle as new sedimentary rock layers. Over time, these may be lifted up from the ocean, to be weathered, taken up, and deposited again. This process is incredibly slow, with phosphorous compounds in the ocean often lasting around 20,000-100,000 years.
Anthropogenic Phosphorus:
Given it’s important role in biologic processes and slow cycling, to facilitate greater rates of product growth, the agricultural industry adds phosphorous to the soils through fertilizer application. The runoff from this addition flows to the same rivers, lakes, and oceans as in the natural processes, frequently leading to aquatic areas of high phosphorous levels. Other major sources of phosphorous include sewage input (aka the human version of animal waste excretion), the addition of orthophosphate to delay metal pipe corrosion, phosphorous-based detergents in urban runoff, and mining. In urban areas, especially those with less developed sewage systems or water treatment systems insufficient to address the total human input, sewage inputs serve as huge sources for aquatic phosphorous. Depending on the location and level of phosphorous added, each of these anthropogenic inputs can throw off the natural cycle, with significant consequences.
Too much of a good thing
Because of its important biologic role, phosphorous additions will promote the growth of aquatic producers, including plankton and plants, which produce food for zooplankton, fish, and humans. While initially this may be beneficial, with increased productivity resulting in higher fish population and biodiversity, phosphate buildup from continuous loading creates an imbalance in the nutrient cycles within a body of water, resulting in eutrophication. Eutrophication is the enhanced production of primary producers (plankton, algae); in terms of nutrient additions resulting in large-scale productions, you might have heard of these events referred to as “algal blooms”. Decomposition processes for old algae requires the consumption of oxygen and, combined with the blockage of sunlight from the algal growth, creates an oxygen-depleted dead zone. Furthermore, some of the bacteria, cyanobacteria, can be toxic to animals, fish, wildlife, and humans that drink or are exposed to the contaminated water. As such, water with excess phosphorous that hasn’t been treated can have significant negative impacts on water quality and biotic health.
Nitrogen and phosphorous inputs increase plankton growth, resulting in eutrophication. Image source: Lincoln, NE Watershed Management
In terms of fish and habitat health, there is no direct physiological impact of orthophosphate on fish. However, higher orthophosphate concentrations combined with warm and sunny conditions promote algae growth which, when decomposing, depletes dissolved oxygen levels in the water. These eutrophication events and algal blooms can significantly damage the biodiversity and quality of an ecosystem, threatening (or killing) fish. Furthermore, other compounds composing runoff from anthropogenic sources- fertilizers, sewage, etc- may contain toxic materials to ecosystems and fish as well. Any other aquatic ecosystem processes involving phosphorous will also be impacted by significant additions of phosphorous beyond the long-term natural timescale.
Common sources of phosphorous in aquatic systems.
Sources and Resources:
Phosphate in Water from Water Research Center
Phosphorous and Water from USGS
The Phosphorous Cycle from Khan Academy
Phosphate versus Orthophosphate from Pediaa
Nutrient Inputs in Watersheds from Lincoln, NE Watershed Management